The Rise of Steel, Part II
Welcome to the Rise of Steel part II. We previously looked at the early stages of industrialization of iron and steelmaking, between roughly 1200 and 1850. To briefly recap, making steel was an involved, multistep process. Iron would first be smelted from iron ore in a blast furnace, resulting in high-carbon pig iron. This pig iron was then placed in a special furnace (initially a finery furnace, later a puddling furnace) to remove the carbon and other impurities, resulting in wrought iron. Wrought iron bars would then be placed into clay chests next to sources of carbon and heated for a period of several days, allowing the iron to gradually reabsorb carbon, producing “blister steel.” The methods varied in their specifics across time and place, but this was the general process in western Europe.
Gradually, the various steps in this process improved. Blast furnaces got larger and more fuel-efficient. Labor and timber-intensive charcoal was replaced as a blast furnace fuel by coke (made from coal). Charcoal-fueled finery furnaces were replaced with coal-fueled puddling furnaces. Steam engines replaced waterwheels for driving bellows and other machinery. Tilt hammers were replaced with faster rollers for shaping the iron into bars and plates.
The replacement of charcoal (which demanded huge inputs of wood) with coke enabled a huge increase in iron production. [0] In 1720, British blast furnaces were producing 13 tons of iron a week on average. By 1806, they had reached 36 tons, and by 1849 average weekly output was 67 tons. Similarly, in 1720, British iron production was just over 20,000 tons a year. By 1806, it had reached 250,000 tons, and by 1850 it had reached 2.25 million tons.
However, this iron was largely turned into wrought and cast iron, not steel (the proportions of which varied over time, but generally most pig iron was converted to wrought iron). The process of producing steel had been improved with Huntsman’s crucible process [1], but that still relied on slow and expensive blister steel as an input. By 1850 steel remained 4 to 5 times as expensive as wrought iron, and was largely used in small quantities for specialized applications such as “cutting tools, files, cutlery, surgical instruments and razors". Of the 2.25 million tons of pig iron produced in Britain in 1850, only 50,000 tons, just over 2%, became steel. Likewise, in the US in 1850 pig iron output was perhaps 560,000 tons a year, very little of which became steel - as late as 1867 only 20,000 tons of steel were produced in the US, less than 2% of the pig iron produced.
The Bessemer process
The desire for cheaper, more abundant steel inspired many people to explore better ways of producing it, but success didn’t come until the 1850s, with Henry Bessemer. Bessemer was a professional inventor, whose previous successful inventions had left him wealthy (highlights include a dated stamp for the Internal Revenue Office and methods for making embossed velvet and bronze powder by machine). He had no metallurgical background, but in 1854 during the Crimean War (and the resulting demand for armaments), he began to study the problem of making better quality iron and steel for heavier guns. During his experiments, he hypothesized that if a large enough surface of molten pig iron could be exposed to air, it would quickly be turned into wrought iron.
Bessemer tested his idea in a small crucible with 10 pounds of molten pig iron. After 10 minutes of blowing air through the iron, he found that it had become wrought iron. Bessemer then repeated the experiment at a larger scale. He built a 4-foot tall cylinder that held nearly 800 pounds of molten pig iron, with openings at the bottom for blowing in air. When Bessemer ran his experiment again, after 10 minutes of blowing air flames erupted from the top of the cylinder. According to Bessemer "Then followed a succession of mild explosions, throwing molten slags and splashes of metal high up into the air, the apparatus becoming a veritable volcano in a state of active eruption."
The explosions resulted from exothermic reactions between oxygen in the air and the silicon, manganese, and carbon in the iron [2]. Not only were impurities in the iron removed, but the process required no fuel input beyond that required to initially melt the iron. Because the melting point of iron rises as impurities get removed, previous methods of iron refining (such as puddling) had resulted in a pasty, partially melted ball of iron as its melting point rose above the temperature of the furnace. But the exothermic Bessemer process produced enough heat to keep the iron liquid as it was refined. Not only did this reduce the labor required compared to puddling (in which workers manually stirred the pasty ball of iron), but it meant that iron and steel produced by the Bessemer process could be cast into large elements. Conventional wrought iron could only produce large elements by welding together individual bars [3].
Bessemer presented his findings in 1856 in a talk to the British Association for the Advancement of Science, titled “The manufacture of malleable iron and steel without fuel.” Within two weeks, he’d sold his first license for the process. But Bessemer licensees almost immediately ran into problems - the metal they produced was found to be brittle, and impossible to forge or roll into useful shapes. One licensee stated that the resulting iron ingots "were crushed into rough gravel like powder, showing a total want of malleability.” Bessemer was forced to refund £32,500 worth of licensing fees.
Two problems vexed Bessemer. First, the air blast left oxygen dissolved in the metal. This was solved by Robert Mushet, who added "spiegeleisen," a cast iron with a high manganese content, to the liquid iron after the air blow was completed (Barraclough 1981). (Mushet also solved the problem of how to reliably get steel from the Bessemer process, by blowing the air until all the carbon in the iron was removed, and then adding it back via the spiegeleisen.) The second problem was the phosphorus content of the iron ore. In his experiments Bessemer had, by chance, used some of the only ore in Britain that was low in phosphorus. Higher phosphorus ores resulted in metal that was unusable. Phosphorus content was less of a problem in wrought iron production due to the lower temperatures, which prevent the phosphorus oxides from reducing back into the metal.
(The problem of high phosphorus ores wouldn’t be solved until 1877 with the development of the Thomas-Gilchrist process, which substituted a dolomite lining for the sand lining in the converter. The basic dolomite lining would react with the acidic phosphorus oxide in the liquid iron, producing slag that could be removed. This version of the Bessemer process would also be known as the basic Bessemer process.)
His credibility gone, Bessemer was forced to open his own steelworks in Sheffield in 1858. Over the next two years he worked to find sources of low phosphorus ores [4], found a more durable converter lining, and developed the iconic pear-shaped “tilting converter” [5]. By the early 1860s his steelworks were turning a profit, and after a successful demonstration of Bessemer steel at the 1862 London International Exhibition, the technology began to be noticed and adopted by other ironmakers. By 1865, steel was being produced by the Bessemer process “for about the cost of wrought iron”, and by 1873 the Bessemer process was producing 500,000 tons of steel a year in Britain, compared to 3 million tons of wrought iron. But although Bessemer steel could substitute for wrought iron, it was of lower quality than crucible steel - it had substantially less tensile strength, for instance - and crucible steel would continue to be used for tools and other applications where higher quality steel was required [6].
The Bessemer process was brought to the US by Alexander Holley, a railroad engineer. On a trip to Europe in 1862 to investigate European weapon-making practices, he visited Bessemer’s plant in Sheffield. Impressed, upon returning to the US he organized a group of investors to purchase a license, and the first Bessemer plant in the US (designed by Holley) was built in Troy, NY in 1865. Holley would go on to design 11 of the first 13 Bessemer plants in the US, and develop many improvements to the process, including a removable bottom that made changing linings faster, and a system for recycling the waste heat emitted by the reaction (Misa 1995). By 1870 Bessemer steel was 38% of the steel made in the US, and by 1875 it was 88%. As in Britain, adoption of the Bessemer process greatly increased the total amount of steel produced. Of the 1.66 million tons of pig iron produced in the US in 1870, there were 69,000 tons of steel. By 1875 that had reached 2 million and 390,000 tons, respectively.
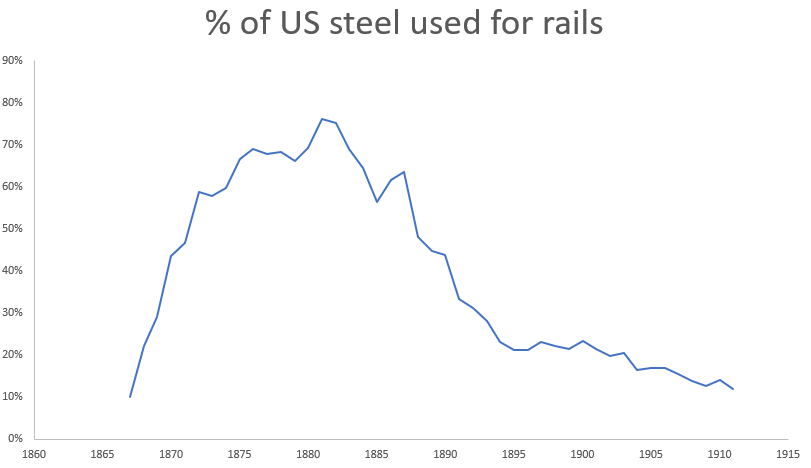
In the US, Bessemer steel was overwhelmingly used to produce a single product - railroad rails. Of the 390,000 tons of steel produced in the US in 1875, 260,000 tons (66%) went to steel rails. By 1879, over a third of all iron and 2/3rds of the steel in the US was used to produce rails. Previously, rails had been made of wrought iron bars welded together and then rolled into a T-shape. These welds created planes of weakness, and wrought iron rails would eventually fail by delaminating along the welds. As railroad track miles increased (by 1880 there were over 90,000 miles of railroad in the US, and rails got heavier to accommodate larger locomotives, railroad companies were motivated to find a longer-lasting rail. US railroads began to experiment with steel rails in the early 1860s, and found that a steel rail would last anywhere from twice as long to twenty times as long as a wrought iron rail [7]. By 1883, Bessemer steel rails were cheaper than wrought iron rails, and had completely replaced iron rails in new and replacement track.
The open hearth process
But while the Bessemer process could be used to produce a satisfactory rail, in the US it struggled to produce steel suitable for other applications such as buildings and bridges. The designer of one of the first steel railroad bridges in the US, the 1879 Kinzie street bridge, described its Bessemer steel as “a rather unsatisfactory material” (the bridge would be dismantled in 1899). Bessemer steel ropes on the Brooklyn bridge were repeatedly found to be brittle, and Bessemer steel beams were known to snap in two or otherwise fail unexpectedly. One engineer in 1887 stated that “The Bessemer process…has also fallen into considerable disrepute for structural purposes. Not a few experienced engineers are now stipulating in their specifications that Bessemer steel will not be allowed to be used, especially for tension-members.”
Even with its updates, several problems with the Bessemer process remained. While the Thomas-Gilchrist modification to the converter lining could remove phosphorus, it (perhaps ironically) required a minimum amount of phosphorus to work. Iron ores in the US, while they had phosphorus impurities, didn’t have sufficient phosphorus content to “make the chemistry go,” and the Thomas-Gilchrist process was largely not adopted here. Bessemer steel thus often had phosphorus impurities that made it brittle. And because the Bessemer process ran so quickly - the reactions ran in roughly 10 to 20 minutes - it was extremely difficult to control.
The Bessemer process would eventually be superseded in the US by another steelmaking process - the open hearth process. The open hearth process was developed by the Siemens brothers in the 1860s (largely by William Siemens), and in some ways was an evolution of the puddling process for making wrought iron. Iron would be placed in a furnace, and a mixture of air and gas would be burned and blown over the top of the iron. The burned exhaust would then flow through a series of brickwork passages, which would absorb its heat. After a period of time, the flow would be reversed, and air and gas would be blown through the now-heated brickwork, absorbing its heat before being burned and blown over the iron. Like the puddling process, the air blowing over the iron would oxidize the carbon and other impurities in the iron [8]. This “regenerative furnace” (first developed by the Siemens brothers in the 1850s) not only used less fuel than a traditional furnace, but also produced enough heat to keep the iron molten even as the removal of impurities drove its melting point up. Thus, like the Bessemer process, it removed the need for manual stirring of the iron. The concept of melting steel in a shallow open hearth had been considered as far back as 1722, but until the development of the regenerative furnace it wasn’t possible to generate enough heat to do it.
On the surface, the open hearth process does not seem obviously superior to the Bessemer process. Unlike the Bessemer process, the open hearth process required fuel to run. It also took much longer than the Bessemer process - several hours, as opposed to 20 minutes - and was significantly more expensive.
However, the longer process made it easier to adjust the makeup of the steel being produced and produce different types of steel. And the fact that the heat from the Bessemer process was generated from the reactions taking place (and thus didn’t require additional fuel) meant that the types of iron it could take as an input were limited. In particular, the Bessemer process couldn’t use as much steel scrap in the mix. As steel scrap prices fell from wastage during steel rail production and from accumulation of worn-out steel rails, its use became increasingly attractive [9]. Peter Temin has also argued that the lower air exposure of open hearth steel compared to Bessemer resulted in fewer embrittling nitrogen impurities. And while the Thomas-Gilchrist process didn’t work on the low phosphorus US ores in a Bessemer converter, the same adaptation (a basic dolomite furnace lining) in an open hearth furnace did allow the use of low phosphorus ores [10]. This ultimately allowed open hearth costs to reach parity with Bessemer costs in the US, as cheaper sources of ore could be used.
Also, by the 1880s, the minimum amount of steel production a Bessemer plant required to produce cheaply enough to compete with established producers was enormous - over 100,000 tons per year. The minimum efficient size of an open hearth furnace, on the other hand, was much smaller - 10,000 tons per year - meaning access to capital was less of a barrier to entry.
Like Bessemer, Siemens encouraged adoption of his process by producing steel in his own steelworks. After a display of open hearth steel at the 1867 Paris Exhibition, the process began to gain traction with ironmakers. While open hearth steel was initially more expensive than Bessemer steel, its other benefits made it more attractive for other uses. In the US, this was at least partly due to the way the American steel industry had developed, with a focus on production volume (particularly steel rail production) that often came at the expense of quality:
As the captive of the railroads the Bessemer process had served their need for large output, but as it turned out this large output was “one of its inherent dangers.” Bessemer mills in the United States could not properly manufacture structural steel for four related reasons. First, many companies had added structural mills alongside their rail mills. To keep both mills constantly busy, managers alternately charged their Bessemer-melting furnaces with pig iron suitable for making rail steel and with iron of “the higher qualities” suitable for structural shapes. When these two streams of metal inevitably got mixed up, the product was excellent rails but inferior structural shapes. Second, workers in the Bessemer casting pits, blooming mills, and bloom yards were “trained to get steel out of the way” quickly, often regardless of its precise quality. This also meant that not all defective steel could be identified and rejected. Third, in contrast to the Bessemer converter’s 10-minute blow, the open hearth’s leisurely place allowed the operator to examine and test the metal…Finally, the mentality fostered by the Bessemer works made it impossible to manufacture high-grade structural steel there…”causes of failure in steel…have proved much more likely to occur at Bessemer mills, where all hands are trained for output and tonnage, than at the open-hearth blooming mills, where the men are more conservative and slower in their work and are less pushed.” - Thomas Misa, A Nation of Steel
As buildings, bridges and infrastructure began to replace rails as the major consumers of steel in the US at the end of the 19th century, steel production was increasingly done in open hearth furnaces. Andrew Carnegie, an early producer of Bessemer steel, would lament that “Engineers are all specifying for open hearth steel. It is impossible to sell Bessemer steel for bridges, boiler plates, ships, or even for these enormous 22-story steel structures which are going up throughout the country”. In 1879, Bessemer steel was 90% of all steel produced in the US. By 1900, it was 66%, with 33% open hearth. By 1911, open hearth was 66%, with Bessemer just 33%.
Increasing scale
The other major development in iron and steel production in the second half of the 19th century was the continuously increasing scale of the process. In 1850, British blast furnaces were producing in the neighborhood of 3500 tons of steel per year. By 1900, US blast furnaces were producing more than 10 times that on average. This was a combination of the blast furnaces themselves getting physically larger (British blast furnaces increased in height from 35-50 feet in 1815 to 100 feet after 1860, and from increasing the amount of iron they could process. The use of hot blast, for instance, not only increased fuel efficiency, but allowed a blast furnace to increase output, as it shortened the process time. As the volume of iron produced exploded in the 1870s, innovations enabling increased throughput of blast furnaces followed:
…Carnegie’s blast furnaces - Lucy, Isabella, and then those at the ET Works - were the largest and most energy-consuming in the world. By “hard driving,” though the use of more intense heat and improved and more powerful blast engines, the Lucy furnace increased production from 13,000 tons in 1872 to 100,000 tons in the late 1890s. By 1890, other furnaces besides those of Carnegie were producing over 1000 tons a week - an enormous increase over the 70 tons a week of the blast furnaces even as late as the early 1870s - Alfred Chandler, The Visible Hand
Despite the massive increase in output, the number of blast furnaces decreased over this time period, as output was concentrated in a smaller number of larger, higher output furnaces. (Today, large blast furnaces produce upwards of 3 million tons of pig iron a year, more than 100 times than all of England produced in 1720).
As scale increased and methods of recycling waste heat were adopted, blast furnaces were increasingly efficient in their use of coke. In 1800, A British blast furnace required 6 to 7 tons of coke for each ton of iron produced. By 1870, that had been reduced to 2 tons, and by 1900 it was approaching 1 ton.
This increase in scale also took place in the rest of the iron and steelmaking process. This first took place in rail mills, which in the 1850s were producing enough rails that they could consume the output of 2 or 3 blast furnaces. By the 1860s several large rail mills with integrated blast furnaces had appeared, which soon began to produce wire, beams, and bar iron. At the time, iron production still relied on the labor intensive puddling process, and these works often employed thousands of people.
These large integrated mills were the first in the US to adopt the Bessemer process in the 1860s, and the open hearth process in the 1880s. Over time, Bessemer converters increased in size (from 2.5 tons in the 1860s to more than 10 tons in the 1880s, compared to 500 pound capacity of the puddling furnace), and steelworks added more of them, often mixing the outputs of several different converters to achieve a more uniform product. By the 1880s, Bessemer plants were utilizing the output of several blast furnaces to feed many converters, and producing more than 100,000 tons of steel a year. Open hearth furnaces likewise increased in size. In 1874, Siemens’ open hearth steelworks was producing 1000 tons of steel a year, or roughly 19 tons per week. By 1900 a single open hearth could produce 40 tons in a single heat. And by the 1950s, hearths of 500-600 tons were being built. Unlike the puddling process, the Bessemer and open hearth processes could be scaled up without adding much labor, and over time the capital/labor and the output per employee increased [11].
The rest of the steel process was similarly improved. As Peter Temin notes, at rolling mills:
Steam and later electric power replaced the lifting and carrying action of human muscle, mills were modified to handle the steel quickly and with a minimum of strain to the machinery, and people disappeared from the mills. By the turn of the century, there were not a dozen men on the floor of a mill rolling 3000 tons a day, or as much as a Pittsburgh rolling mill of 1850 rolled in a year. - Peter Temin, Iron and Steel in Nineteenth-Century America
And Smil notes that the coke-making process also improved:
…before 1900 about 95% of [coke] production had been done in closed behive ovens…they discharged distillation and flue gasses through a central chimney, and the heat required for pyrolysis was supplied by partial combustion of coal, an inefficient process that wasted about 45% of the charge fuel…Otto-Hoffmann regenerative by-product ovens…where chemicals and energy in waste gases are recovered while coke yields are increased, became the mainstay of modern coking…Their coke yield (as share of the charged coal) is higher than in beehive ovens (commonly 10-15% and they work with a variety of bituminous coals. - Vaclav Smil, Still the Iron Age
By the end of WWI, 50% of coke in the US was being produced in by-product ovens.
As steel mills got larger, more mechanized, and increasingly efficient in material and energy use, the costs of producing steel fell. In 1867 a Bessemer steel rail cost $167 in the US, roughly twice as much as a wrought iron rail (already an improvement compared to the crucible process). By 1898 it had fallen to $17.62, a price at which the largest, most efficient producers were still earning a gross profit of nearly 40% [13], And though wrought iron continued to be used (see the wrought iron Eiffel Tower in 1889), steel increasingly replaced it. By 1906, virtually all pig iron was being converted into steel [13].
Sources
(Roughly in order of importance)
Thomas Misa, A Nation of Steel
Peter Temin, Iron and Steel in Nineteenth-Century America
Kenneth Barraclough, The Development of the Early Steelmaking Processes (thesis)
Jean McHugh, Alexander Holley and the Makers of Steel
Vaclav Smil, Still the Iron Age
R.F. Tylecote, A History of Metallurgy
Alfred Chandler, The Visible Hand
Alfred Chandler, Scale and Scope
Robert Rogers, and Economic History of the American Steel Industry
Jeremy Atack and Jan Brueckner, Steel Rails and American Railroads, 1867-1880
Carnegie Steel, The Making, Shaping and Treating of Steel (Also later editions by US Steel)
Alan Birch, The economic history of the British iron and steel industry, 1784-1879
Alexander Holley, A Treatise on Ordnance and Armor
Deirdre McCloskey, Economic Maturity and Entrepreneurial Decline
Footnotes
[0] - This appears to be less true in places that had an abundance of timber. The US, for instance, continued to use charcoal long after Britain had switched to coke (Temin 1964).
[1] - Steel could also be produced directly from the puddling process, but most steel was still produced via cementation (Barraclough 1981).
[2] - More specifically, the reactions that took place are described by Barraclough:
[3] - For instance, here’s Holley discussing steel in 1865:
The grand advantage of low [carbon] steel over wrought iron, for nearly all purposes, is that it can be melted at a practicable heat and run into large masses; thus avoiding the serious defect of wrought iron in large masses - want of soundness and homogeneity...The want of homogeneity - the numerous stratea of impurities and plans of weakness introduced into wrought iron, especially in large masses, all the way from the puddle-ball to the finished gun…its grand defect, by the present processes of manufacture, is imperfect welds. The casting of low steel into masses of any size overcomes this whole difficulty.
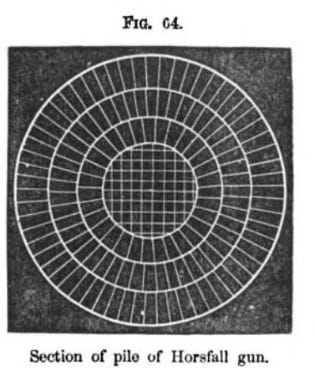
A wrought iron cannon, for instance, would be built up from many wrought iron bars welded together:
Cross section of a wrought iron cannon showing individual bars
[4] - The role of phosphorus was not understood until the early 1860s, and finding suitable ores was hit or miss until then (Misa 1995).
[5] - The purpose of the tilting was so that the liquid iron would not run out through the airways after the air blast was complete (Misa 1995).
[6] - Later crucible steel could use Bessemer steel as an input, and didn’t require cemented blister steel first. In the early days of Bessemer’s steelworks, its main product was crucible steel (Misa 1995).
[7] - Railroads experience with steel vs iron rails varied, as can be seen in this table from Atack 1982:
[8] - The chemical reactions that took place in the Open Hearth furnace were largely the same as took place in the Bessemer converter (Barraclough 1981).
[9] - There were, broadly, two variations of the open hearth process - one which made steel from pig iron and scrap steel, and one which made steel from pig iron and iron ore. These were sometimes known as “pig and scrap” and “pig and ore”, respectively. The “pig and scrap” process was also known as the Siemens-Martin process. (McHugh 1980).
[10] - I don’t 100% understand why this is, but I assume it goes back to the self-heating nature of the Bessemer converter requiring a certain ore chemistry to work.
[11] - For some reason different sources (Temin, Chandler, Rogers, Smil) dramatically disagree on the average output of a US blast furnace. I’ve used the Rogers numbers in the above graph.
[12] - Though a spike in demand and then the policies of US Steel would return to a consistent price of $28.00 (Temin 1964, Chandler)
[13] - Though much of it was low carbon “mild” steel that was chemically very similar to wrought iron, simply produced by the open hearth and Bessemer processes instead of puddling. (Tylecote 2002, Misa 1995).










I have a lot of substack subscriptions, but none more happily anticipated than this one. It must be my inner engineer!
I read this and I felt like it was about me ! I'm in the process of building my own steel foundry for my iron ore magnitite. I'm heating it was foundry coke and I have to reach a temperature of 3400 degrees and at 3000 degrees it employees making a sonic boom then the temperature rises so fast with in one hour it's 4000 degrees you have to actually shut it down and I'm pouring gun grade hard steel .2000 pounds of the iron ore crushed I should get 1500 pounds of gun grade steel back. It's assume!